What are liquid crystals?
There are three common states of matter, solid, liquid and gas- are different because the molecules in each state have a different degree of order.
Crystalline solids
Liquid phase
A liquid crystal is a fluid like a liquid, but is anisotropic in its optical and electromagnetic characteristics like a solid. When the liquid crystal is formed from the isotropic state some amount of positional or orientation order is gained. It is this order that accounts for the anisotropies of the substance
Thus liquid crystal may be described as the distinct states of matter in which the degrees of molecular ordering lie intermediate between the ordered crystalline state and the completely disordered isotropic liquid.
In a liquid crystal the molecules possess orientation order, i.e., the molecules tend remain oriented in a particular direction. The direction of preferred orientation in a liquid crystal is called the director. In a liquid crystal phase they spend more time along the director than in any other direction
Liquid crystals are classified into two main categories`
Thermotropic liquid crystals are those, which exhibit liquid crystalline properties as the temperature is varied. The liquid crystal to liquid transition and liquid-to-liquid crystal transitions of a mesogenic material are essentially reversible. The mesophase which are formed by both the heating and cooling cycles are thermodynamically stable are called enatiotropic phases. But in some compounds, their thermotropic phases appears only during the cooling phase from the isotropic phase, but not on the heating process. These mesophases, formed by the super cooling of the material below its melting point, are meta-stable and are called monotropic phases.
Thermotropic liquid crystals can exist in three phases
|
|
|
Isotropic |
Nematic |
Smectic |
They exhibit long range molecular ordering but possess no positional ordering. The preferred direction varies from point to point in the medium, but there is a uniform alignment with respect to the long axis- director. The nematic phase is bifringent due to the anisotropic nature. They also exhibit dielectric anisotropy

The cholesteric phase
In this structure the local molecular ordering is identical to that of the nematic phase, but are exhibited my molecules possessing chiral centers. Chiral nematic liquid crystals are also refereed as twisted nematic liquid crystals. Unlike in nematic phase where all the molecules stay parallel to one another, in chiral nematic phase, the molecules arrange themselves in such a way that a group of molecules align at different angles with respect to their adjacent groups i.e., the director is not fixed in space, but rotates through out the sample forming a helical pattern as it changes its direction. The distance traveled by the director as it completes one full turn is called the pitch of the liquid crystal. The twist present in chiral nematic crystal makes them to exhibit spectacular optical properties, which is made use of in displays. The most striking features of cholesteric mesophase is strong optical activity and selective light reflection. The pitch is temperature dependent and hence cholesteric phase is finding application in thermography

Substances that form smectic phases are soap-like. In smectic mesophase, there is a small amount of orientational order and also a small amount of positional order. The molecules tend to point along the director and arrange themselves in layers. Based on the orientation of the director there are many types of smectic phases. The interlayer force of attraction in smectic phase is weak as compared with the lateral forces between molecules. As a consequence the layers are able to slide over one another.

Liquid crystals formed by molecules, which have disc like or plate like structures are classified as discotic liquid crystals. The simplest discotic phase is also called the nematic discotic phase because there is oreintational order, but they lack positional order. There is random motion of the molecules but on an average the axis perpendicular to the plane of each molecule tend to orient along the director. In smectic discotic or columnar phase, in addition to the oreintational order present, most of the molecules tend to position themselves in columns. The columns are arranged in a hexagonal lattice resembling a set of coins stalked into a pillar
Chiral smectic
In a similar way to chiral nematics there are chiral forms of smectic phases. The tilted director rotates from layer to layer forming a helical structure.
![]()
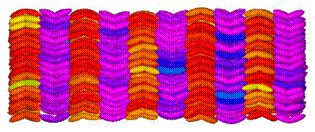
Apart from the cigar shaped molecules some more exotic shaped liquid crystals are also reported. Below figure illustrates the stacking of banana shaped liquid crystals.
LC Phase |
Example |
Nematic Phase |
|
Cholesteric Phase |
|
Smectic A |
|
Smectic B |
|
Smectic C |
|
Smectic D |
|
Smectic E |
|
Smectic F & G |
|
Smectic H |
|
Discotic |
|
Banana Shaped molecules |
|
Liquid crystalline behaviour depends on the rigidity at the center position of the elongated molecule. If the molecule lacks rigidity, bending may occur along its lengths destroying parallel arrangement of molecules resulting in no crystal formation
Long chain n-alkanes have elongated conformations, but the flexible alkyl chain can coil and bend and hence no liquid crystal phase can be formed. On the other hand introduction of a double bond makes the formation of mesophase molecules as the conjugates part makes the molecule rigid
E.g.: introduction of alternative double bonds
Aromatic compounds are polarizable, have a planar structure and are rigid. Introduction of alkyl groups in the suitable positions make the molecule elongated and hence such compounds can exhibit mesophase. The requirement for liquid crystal formation in compounds with benzene rings is
The central group may also involve a ring formed by dimerization through hydrogen bonding, which makes it rigid.
![]()
![]()
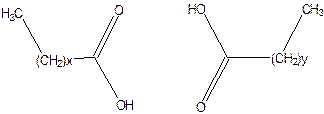
n-alkanoic acid mesophase not possible
![]()
![]()

If the benzene rings are linked through para subtituents, mesophase formation is favoures. But linkage through meta or ortho positions is not favorable. However, introduction of an additional meta or ortho substitution to an existing para substituted molecules favours mesophase formation.
The most common rigid cores, bridging groups and terminal groups found in rod-like mesogens are given below

A & B HEXANE or AROMATIC RING
R ALKYL SIDE GROUP
Z LINKAGE GROUP
X TERMINAL GROUP
A series of compounds of the same type wherein successive members differ in their formula by a –CH2 group are called homologues series. The thermal stability of the molecules of members of a homologues series changes with the number of carbon atoms in the chain
The liquid crystal phase of p-azoxyanisole (PAA) is stable between 118 to 1350C. PAA has more than 12 homologues, which are formed when –CH2 groups are added to its side chain.
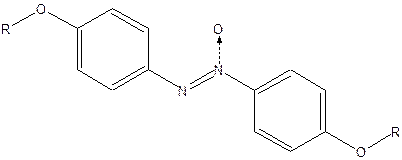
It can be seen that in general the transition temperature of the liquid crystal decreases with the increase in the number of carbon atoms in the side chain. However, there is an odd-even distribution of transition temperature and was found to decline with increase in number of carbon atoms. When the alkyl group side chain contains one to six carbon atoms liquid crystals show a nematic phase and when the number of carbon atoms is greater than six, smectic phase is observed. Homologues containing seven and eight carbon atoms, however, show a transition from solid to smectic to nematic before melting to a liquid.
The high transition temperature of PAA series owing to the alkyl side group linkage through oxygen atom makes them unsuitable for display applications. If alkyl groups are attached directly to the benzene, p-alkylazoxybenzene series with lower transition temperature is obtained.
Another example of liquid crystal homologues having a low transition temperature is p-methoxybenzylidine, p-n-butylaniline (MBBA) series.
Altering the flexible side chain can alter the transition temperature of liquid crystal phase. Biphenyl and terphenyl systems carrying highly polarizable groups such as nitro and cyano groups are also show lower transition temperatures.
Nematic liquid crystals are the simplest forms with rod like molecular structure and align themselves spontaneously along the director
Dielectric anisotropy (De) is defined as the difference between the dielectric constant parallel and perpendicular to the director. The optical anisotropy (Dn) is defined as the difference between the refractive indices parallel and perpendicular to the director. These two properties are important for the electro-optic effects in liquid crystals
The director in a liquid crystal is free to point in any direction. But when a film of liquid crystals is placed between two plates of certain materials, the director is forced to point along a perpendicular direction. When a thin film of liquid crystal is sandwiched between two glass plates, the molecules close to the glass surface are forced to orient themselves parallel to the surface of the glass sheets.

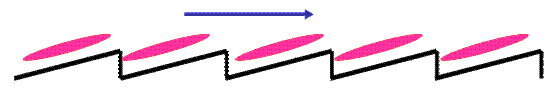
In the absence of an electric field (below a threshold value) the directors at other layers are also aligned parallel to the surfaces giving a homogeneous texture. But when an electric field is applied perpendicular to the molecules near the center of the crystal orient themselves along the applied field. The deformity begins at a threshold vale of the strength of the applied field and increases with increase in the strength of the field. This deformity brings about a significant change in the optical characteristics of the liquid crystals and are made use in liquid crystal displays
|
|
|
|
Original orientation |
Situation in electrical field |
Result electrical field |
Result strong electrical field |
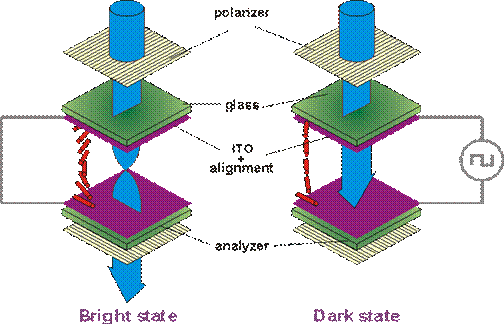
When light is incident on two crossed polarizes no light emerges as the light coming out of the first polarizer is completely absorbed by the second polarizer and hence appears dark. When a pair of crossed polarizers is filled with a twisted nematic liquid crystal having a positive dielectric anisotropy, the twisted structure acts like a wave-guide and gradually rotates the plane of polarization of light by 90o . Hence a linearly polarized light incident on the cell emerges linearly polarized but in an orthogonal direction resulting in a bright appearance. The 90o twist in the cell is lost when a sufficiently strong electric field is applied to the cell. Hence the cell appears to dark between the two crossed polarizers. However the following conditions should be met to see the elelctrooptic effects
Dn x P > l
(i) DISPLAYS
All information displays utilizes the ability to control light, in order to function. By controlling what parts of a display are bright and what parts are dark, information is passed to the user. Regardless of the complexity of the display, the basic working principle remains the control of light from small area of the display. This can be done in two ways- active displays and passive displays
E.g.: cathode ray tube (CRT), light emitting diode (LED)
E.g.: liquid crystal display (LCD)
In LCDs in order to enhance the difference in the brightness between dark (turned off) and bright (turned on) areas. Dichoric dyes are used to give desired coloures to the display with a good contrast
Liquid crystals can be used to measure temperature utilizing the selective reflection property of chiral nematic liquid crystals when a light is incident on a cholesteric mesophase parallel to the axis of rotation of the helix, radiation of very small wavelength range corresponding to
n x P = lR
gets divided into two beams one of the beam is reflected and the other is transmitted. If the wavelength range lR is in the visible region, the reflection give rise to light colours. The lR and hence the colour of the reflected light depends on the temperature of the mesophase. Temperature dependence of the colour reflected by liquid crystals has been utilized for application in thermography.
(iii) DETECTION OF AIR POLLUTANTS.
It has been observed that the colour of liquid crystals changes in the presence of impurities. When impurities diffuse in to the cholesteric liquid crystal film, the pitch is altered and hence the colour changes. This property is used in the detection of contaminants in the atmosphere.
(iv) Solitary wave propagation
A high intensity laser beam injected in a liquid crystal can produce a local reorientation of the director molecules. In this way the light produces its own wave-guide and the laser beam will not diffract but stays confined in a narrow beam. The soliton application can lead to an addressable liquid crystal wave-guide to switch light between several optical fibers.
Source: https://thevtu.files.wordpress.com/2009/01/liquid-crystals.doc
Web site to visit: https://thevtu.files.wordpress.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes