In this section you will learn about:
Metals
Metals have been used by humans for over 6000 years. The first metals were simply picked up off the ground, but in time people learnt to extract metals from their ores. Nowadays the technology has become quite complex and not only can many metals be extracted from their ores, but the properties of metals can be modified by various types of finishing processes or by mixing with other metals to form alloys. For building purposes, most metals are alloys.
The major base metals used are iron, copper, lead, zinc and aluminium. Metals using iron as their base are called ‘ferrous’ metals while the others are termed ‘non-ferrous’. Brass is an important non-ferrous metal used in building, being an alloy of the base metal copper.
Glass today is manufactured from the same materials as it was several thousand years ago. Egypt is credited with the earliest glass making technology at least as early as 4000 BC. In Australia commercial glass making began in 1872 in Melbourne with bottle manufacture. In 1903 factories were established and in the 1920s and 1930s products were increased to include window glass. Glass is manufactured commercially from sand (silica), soda and lime. Its characteristics are dependent on the proportions and treatment.
Metals are substances that can either be hammered (the quality called malleability) or drawn out as wire (the quality called ductility) or melted and formed into shapes in moulds. Most metals can be polished. All metals are, to greater or lesser degrees, conductors of forms of energy such as heat and electricity.
Other characteristics possessed by metals may vary considerably from metal to metal. Some metals (eg. stainless steel) have good strength qualities, whereas others (eg. tin) have very little strength. All metals, however, will lose strength when repeated force is applied to them, this is a process known as metal fatigue.
The degree of hardness of a metal will vary according to its natural characteristics (lead and tin, for example, are soft metals; chromium and nickel are hard) and according to the degree to which the metal is worked. When a metal is worked at normal temperatures (by being rolled or forged, for instance) the result will be an increase in its hardness and strength, this is called work hardening.
Most metals are subject to corrosion, which occurs when the surface of the metal combines with oxygen in the air to form a coat or crust that is no longer metallic (eg. rust on iron or steel). Corrosive liquids and gases can actually eat away metals. (We can see the effect of salt air or spray on aluminium.)
The process of corrosion is usually greatly sped up by the action of heat and moisture. Some metals have very low corrosion-resistance, while others have a good degree of corrosion-resistance. Metals with a high degree of corrosion resistance (eg. chromium) are often used either as coatings or in alloys with other metals to increase their resistance to corrosive agents.
How metals are formed depends upon the type of metal, the objects being made, and whether parts made of other metals are also incorporated. The following are some of the methods used:
Metals can be joined by a variety of methods, including the following
These are joint that are held together by additional fixings such as bolts, screws or rivets. There is no adhesion between the pieces that are joined together.
Most metals can be joined using an alloy called solder. Solder is a mixture of two or more metals that melt at a lower temperature than the melting point of the metals being joined.
Solder usually refers to tin-lead and lead-silver alloys which melt below 300°C and is applied using a ‘soldering iron’.
“Silver solder” is another alloy but with a higher melt temperature and is applied using a ‘oxy’ torch (therefore considered a brazing).
Because solder contains lead it cannot be used on water pipe, silver solder is used instead.
Brazing gives stronger joints than soldering because it is done at higher temperatures (over 600°C) using an ‘oxy’ torch.
Brazing cannot be used on metals such as lead which have low melting points.
Originally, most welding involved a metal being heated to a temperature below its melting point, and the soft metal being hammered together. This traditional blacksmithing method has been replaced by gas welding (using oxyacetylene or propane) and arc welding (using an electric arc struck between the work and a welding rod or a carbon electrode).
Modern welding involves heating the adjacent metal to extremely high temperatures which allows the metal to flow together and form one continuous unit with the addition of a filler metal.
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………
Ferrous metals are those metals that contain a large amount of iron. The main types of ferrous metal are:
Iron ore, as mined, is a combination of iron and oxygen and various other substances. In this country most of the ore is obtained from open-cut mines.
The first step in processing the ore is to reduce it to metallic iron (often called ‘pig iron’), a process carried out in a blast furnace using coke as a fuel and reducing agent. The metallic iron, at this stage, contains a relatively high proportion of carbon (about 4%).
To make steel, the carbon content of the metallic iron must be lowered to less than 1% by an oxidation process in the steel-making furnace. At the same time, the metal is given whatever special chemical and physical properties may be required by the addition of other metals.
The quantities and timing of the additions of carbon and various other elements are carefully controlled to make the wide range of irons and steels that are available.
Carbon is the principal hardening element in steel. In plain carbon steels, it is used as the controlling element to regulate physical properties. When the carbon content is increased, hardness and tensile strength are improved but ductility and weldability are reduced (refer to Figure 1 on the next page).

Figure 1: Influence of carbon on the properties of ferrous metals
Manganese increases strength and hardness but to a lesser degree than carbon. It also improves the toughness and abrasion resistance of steel.
Chromium increases hardening ability and tensile strength and improves corrosion and abrasion resistance. It is usually associated with nickel additions to form ‘stainless steel’.
Blast furnace slag is the waste from the smelting process. It is an important by-product which can be used for concrete aggregate, road metal and slag wool for insulation.
This is a major source of metallic iron for steel making. Scrap may either be residue left from the steel making process or purchased from discarded or obsolete constructions. About half of the crude steel produced annually in the world will eventually be returned to the steel-making furnaces.
Cast iron is produced by re-melting pig iron with steel and cast iron scrap. The cast iron has a high carbon content which makes it free-running and, therefore, very suitable for moulding intricate shapes. Cast iron has been used in the past for the decorative iron lace on buildings which is often wrongly called ‘wrought iron’.
Cast iron is used for fire grates; for soil waste pipes and ventilating pipes; for drainage gratings and frames; and for baths and basins (with a vitreous enamel finish).
This is a low carbon iron which is excellent for forging but cannot be cast, tempered or welded (by gas or arc). Wrought iron was very popular for decorative finishes (such as balustrades and balcony railings) in the 1950s but has since lost popularity.
Steels are produced by removing impurities from pig iron and then accurately adjusting the quantities of all the ingredients.
Steels are noted for their high strength compared to their production costs, and also for their poor performance in building fires. Ordinary steels do not resist corrosion well, but special steels (eg. stainless steel) are produced today with excellent corrosion resistance.
Structural steel products are available in hot rolled sections and cold formed sections.
Hot rolled sections are formed while the steel is at elevated temperatures and include solid and hollow sections of:
Refer to Figure 2 on the next page.
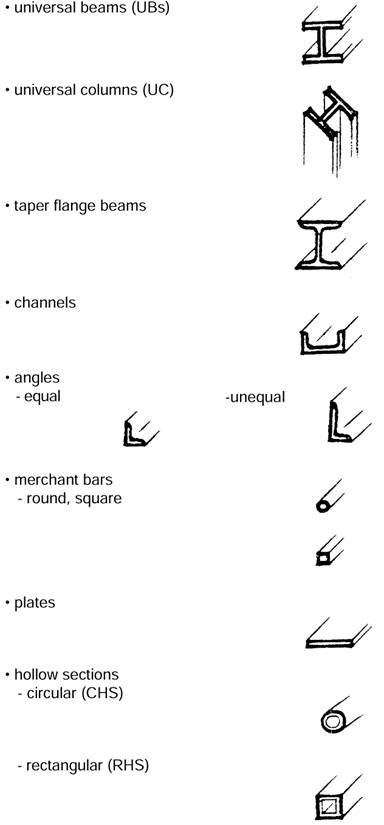
Figure 2: Typical hot rolled steel sections
These are formed while the material is cold as distinct from materials that are shaped or worked while under the effect of heat. Unlike hot rolled sections, cold formed sections have constant thickness.
Cold formed sections may be formed by:

Figure 3: Rolling in a rolling mill

Figure 4: Pressing with a press brake
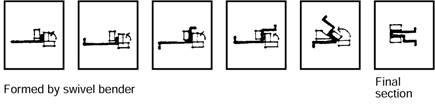
Figure 5: Pressing with a swivel bender
Figure 6 shows some of the specialised (and galvanised) lightweight sections that have been developed for use in residential construction.

Figure 6: Single storey construction showing steel floor and wall framing and steel trusses for tiled roof
These galvanised lightweight sections are manufactured by one of the following methods.
These are used for:
Refer to Figure 7 on the next page.
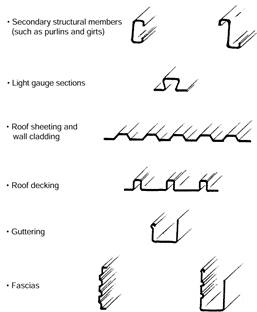
Figure 7: Cold rolled sections
Pressed steel is used for:
Note: Pressed steel sections are limited to the size of the break press; or, with swivel bending, are able to be produced economically in small quantities.
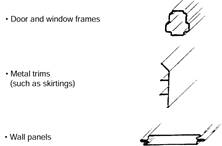
Figure 8: Pressed steel sections
Alloy steels contain certain added elements that provide special properties such as ultra high strength or resistance to corrosion or heat.
Stainless steel (steel containing chromium and nickel) is one such steel alloy which, although much more expensive than mild steel, is being increasingly used in building in a wide variety of applications. This is because of its durability and low maintenance needs, even under extreme conditions of atmospheric pollution, as it has excellent resistance to corrosion.
Stainless steel has outstanding structural advantages because its hardness and toughness allows it to be used in very light sections, thus reducing greatly the weight of finished articles. Even more importantly, it is less affected by extreme heat, such as in a fire.
Except for very simple cutting or drilling on site, all shaping and fitting of stainless steel must be done in suitably equipped factories and workshops.
Stainless steel is also used for sanitary ware (eg. sinks and benchtops).
Upon exposure to the atmosphere, ferrous metals combine with oxygen to form a red oxide (ie. rust). Rust corrodes the metal and eventually wears it away, leaving behind a red powdery residue. This not only affects the appearance of the metal but substantially reduces its strength.
One way of making steel rust resistant is by applying one of many protective coatings available for steel products. These fall roughly into two groups: metallic coatings and non-metallic coatings. As most require scrupulously clean conditions and special surface preparation of the steel for successful application, factory application of surface coatings is preferable.
These function by taking advantage of electro-chemical differences between different metals. In adverse atmospheric conditions it is the surface coating that is sacrificed rather than the base metal.
A variety of methods are used to apply metallic coatings, such as electroplating, spraying and hot dipping. Metals used to coat the steel include cadmium, zinc, tin, aluminium and copper.
Zinc aluminium alloy applied by the hot dip process has effectively replaced galvanised steel in applications such as roofing because of its greatly increased durability.
These are available in a wide variety of colours and include:
Painting should be considered as a complete system that includes surface preparation, pre-treatment to facilitate adhesion, primer, intermediate coat or coats and finish coat. Different types of steel require different pre-treatments and coatings.
Baked epoxy finishes are applied to zinc-aluminium coated steel which is chemically treated to assist bonding. An epoxy primer and then the final colour coat are baked on separately. This type of finish is popular for domestic and commercial roofing and wall cladding for normal conditions.
In marine and polluted industrial conditions steel can be coated with a tough vinyl which is laminated to the steel substrate. The vinyl coating locks out moisture, making an extremely corrosion-resistant finish.
Bituminous coatings are based on bituminous resins such as coal tar or asphalt. The bituminous resins perform well underground and in contact with water but do not have good weather durability when exposed to sunlight.
Vitreous enamel coatings comprise a layer of glass fused to a properly prepared steel base.
……………………………………………………………………………
……………………………………………………………………………
……………………………………………………………………………
……………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………
Door & window frames: ……………………………………………………………………………
Roof sheeting: …………………………………………………………………………..
Fascias: ……………………………………………………………………………
Wall panels: ……………………………………………………………………………
Metal trims: ……………………………………………………………………………
Guttering: …………………………………………………………………………….
Domestic wall framing: …………………………………………………………………………….
…………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………
Most non-ferrous metals are more costly to produce than ferrous metals. However, they often have much better working properties and resistance to corrosion. The more common non-ferrous metals are copper, aluminium, zinc, lead, nickel, tin and cadmium.
Copper has been in use for at least 10,000 years: nearly 5000 years ago it was being beaten into sheets, pipes, and other building products.
Copper is a pinkish coloured metal and is easily hammered into sheets. It is much more expensive than some alternatives but its extreme resistance to corrosion outweighs this disadvantage in certain applications. Upon exposure to the atmosphere, copper forms a protective copper oxide coating which is light green in colour.
Its resistance to corrosion has made it popular for use as water pipes and tanks. It also conducts electricity very well, hence its use for electrical wiring. Other uses include roofing, roof plumbing, flashing and damp courses.
Brass is an alloy of copper and zinc, and is an attractive golden colour.
Brass is used for plumber’s hardware (eg. pipe connectors and fittings; taps and outlet spouts, often chrome finished). Screws, nails, grilles, hinges, door locks and latches and chains are often made from brass.
Aluminium is a lightweight metal (approximately one-third the weight of iron) and is silver-white in colour.
Aluminium was introduced as a building material after the 2nd World War (1939 – 1945) in competition with traditional building metals, such as steel and copper. Probably the major characteristic that has helped aluminium gain widespread acceptance in the building industry is its suitability for extrusion production methods. This means that very complicated shapes can be produced economically.
Aluminium products are extensively used in the building industry.
For example, aluminium is used for
One of the most significant properties of aluminium is its excellent resistance to atmospheric corrosion. On exposure to the atmosphere, a whitish coating of aluminium oxide forms which then protects the surface from further corrosion. The structural integrity is not impaired as a result of this process.
Thus, untreated aluminium can be used for roofing, cladding and so on, but where long-term appearance is important the aluminium should be finished.
Corrosion of a metal may be accelerated through contact with another metal of very different electro-chemical properties especially in the presence of an electrically conductive solution, such as sea spray or industrially polluted moisture.
Copper, brass and nickel alloys, all have a large potential difference to aluminium and in a salt solution cause it to rapidly corrode.
Some other building materials are also incompatible with aluminium and direct physical contact with those materials should be avoided or barriers should be used. Table 1 on the next page broadly indicates the types of barriers suitable for most building construction applications.
Although aluminium is naturally corrosion resistant, various finishes may be applied for aesthetic reasons. These include textured finishes ranging from a fine satin finish (achieved by chemical etching) to a scratch-brushed or hammered finish.
Bright finished aluminium can be achieved mechanically or chemically and results in highly reflective product. To retain the desired appearance, however, the sections should be anodised immediately.
Anodising is an electro-chemical process which greatly increases the thickness of the protective oxide film which would naturally form on the surface, thereby increasing the resistance of the surface to corrosion and damage and enhancing the appearance of the finished product. Film thicknesses can be specified for different applications.
The oxide film may be artificially coloured. Depending upon the process, however, some colours may be subject to ultraviolet deterioration and therefore are only suitable for interior applications.
Paint may be applied to aluminium but factory application is recommended as the process must be carried out in a dust-free environment and the aluminium surfaces must be pre-treated to remove surface contaminations and to provide a key for good adhesion. Powder coating is now widely used as a finish to aluminium in residential building.
Contact material |
Compatibility |
Recommended barrier |
Stainless steel 18/18 type or 300 series should be specified |
Satisfactory. Recommended for all fastenings. |
No protective barrier required. |
Zinc |
Under severe environments such as coastal or industrial, zinc will suffer from preferential attack. |
In severe environments metal contact surfaces should be coated with a bitumastic paint. |
Galvanised steel
|
As for zinc. |
As for zinc. |
Mild steel |
Aluminium will corrode in contact with mild steel in presence of an electrolyte.
|
Coat contact surfaces with bitumastic paint or yellow zinc chromate paint. |
Lead |
Corrosion of the aluminium will only occur in marine or severe industrial environments. |
In severe environments separate contact surfaces with non metallic spacers or bitumastic paint.
|
Copper and brass (including monel metal) |
Attack of the aluminium surface in contact with these materials will occur in most atmospheric conditions. |
Copper and brass must be plated with nickel and/or chromium; otherwise use non-metallic separators. |
Concrete, cement, lime etc, stone and brick |
Wet or ‘green’ products can cause severe attack on aluminium. |
Surfaces in contact with these products must be protected by painting or separating with non-metallic material. Wash thoroughly with clean water if contact occurs. |
Damp or unseasoned timber |
Because of their acidic nature can cause aluminium to corrode. |
Timber must be primed with yellow zinc chromate undercoat and sealed with suitable protective paint. |
Treated timber |
Wood preservatives use salts of heavy metals such as mercury or copper, or certain chlorides. |
Timber should be coated with caulking compound or mastic. |
Hardboard, plasterboard |
The absorption of moisture into hardboard or plaster board may give rise to poultice corrosion. |
Seal using suitable primer. |
Plastics, rubber |
No corrosive effect. |
No special treatment required. |
Adhesives, sealants, etc. |
These should not contain chlorides in excess of 0.1% and those containing water soluble sulphates should be fully tested before use. |
Carefully select adhesives and sealants compatible with aluminium. |
Table 1: Compatibility of aluminium with various building materials
Most physical joining of aluminium elements is achieved with the use of bolts and nuts, screws, nails and rivets. For reasons of compatibility, fasteners are normally aluminium alloy, stainless steel or cadmium-plated steel.
Some modern adhesives such as epoxy and epoxy-PVC types are commonly being used to produce high-strength joints between aluminium and a great variety of other materials.
Welding is also used to join aluminium. If welded assemblies are subsequently anodised, some discolouration in the anodised film occurs across the welded zone.
Zinc is a soft, greyish metal which can be hammered or rolled into sheets: such sheets have been used for roofing rainwater goods. Today, zinc’s most important function in the building industry is as a protective coating on steel.
The zinc coating acts first as a barrier to corrosion. However, should the coating be scratched or damaged, exposing the steel, the zinc surrounding the damaged part will itself corrode instead of the steel. Thus, by sacrificing the zinc, the steel is protected and will not rust until all available zinc is used up.
Research has produced a protective coating for steel which combines zinc and aluminium in an alloy. It is easily applied, by hot dipping, and holds to the metal better than zinc galvanising, thus giving much better protection. It is used on sheet steel and cladding.
Lead is soft and easily worked, but its great density makes it heavy to handle, and thin sheets and pipes will not even support their own weight.
Lead has been used for thousands of years: lead water pipes were used by the Romans, and our word ‘plumber’ comes from the Latin word plumbum meaning lead.
Due to its toxic properties, however, lead is no longer used for water pipes. In the past, it was used for roofing and roof plumbing, but today its use is limited such as where the run-off will be collected and used for drinking water.
However, in certain roof plumbing situations, its weight and malleability still make it a useful and preferred material. Such a case is as flashings on tiled roofs where the water is not used for drinking. Rolled flat-sheet lead rated at 20kg/m² is used for this application because it can be ‘hammered’ to follow the profile of the tiles and wind will not lift the lead.
Lead is used:
Nickel is a hard, silvery-white, malleable metal, resistant to corrosion.
Tin is a very costly, soft, weak metal with a low melting point (232°C), but extremely resistant to corrosion.
Cadmium is a white, malleable metal that looks like tin.
Chromium is well known for its high resistance to corrosion as a plating, and as a constituent of stainless steels and other corrosion-resistant alloys. It is extremely hard and scratch resistant.
Stainless steel is much harder than mild steel and silvery in appearance. It has wide applications in commercial buildings and has been used extensively for domestic sinks. More recently it has been used for bench tops and as a termite barrier where it takes the form of a very fine mesh which termites cannot penetrate.
Corrosion of dissimilar metals can occur when they are in contact with each other, particularly in damp situations.
In building, the selection of metals which will come into contact with each other must be done very carefully. For example, zinc will corrode in the presence of lead; therefore, the use of lead flashes for zinc plated roofs may well lead to corrosion of the roof.
……………………………………………………………………………
……………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………………………………………………………….......................................................................
…………………………………………………………………………………………………………………………………………………………………………………….......................................................................
Residential and commercial buildings can both be of metal frame construction. This type of construction is versatile, light, strong, time and labour saving, economical, and stable. Walls, roofs and floors can all be constructed this way.
The metal frames made from steel are pre-fabricated in the workshop or before being erected. They can be joined together using rivets, welds, screws or bolts.
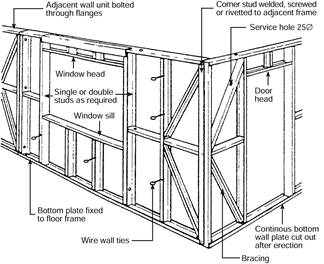
Figure 9: Metal framing for a brick veneer house
The wide range of metal fasteners used to join or fix building materials and components includes:
Nails are manufactured in a wide variety of sizes, shapes and finishes, according to their particular use (refer to Figure 10).

Figure 10: Types of nails
Screws are available in a range of sizes, shapes and coatings for use with wood or masonry.
The four most common types of wood screws are:
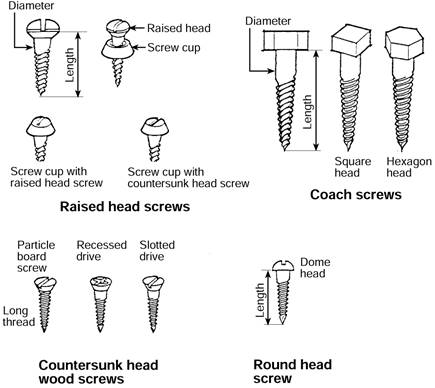
Figure 11: Common types of wood screw
Bolts, nuts and washers are normally made of plain steel, alloy steel or a non-ferrous metal, and may have a protective metal coating (such as zinc or cadmium). The bolt heads are usually either dome headed with a square shank; dome headed with a slot; hexagonal or square headed. The nuts may be square or hexagonal and the washers are flat discs with a central hole.
The two most common types of bolt are:

Figure 12: Two common types of bolt
These are used for joining various timber-framing members. They are made from hot-dipped galvanised steel and are strong and quick to install. Figure 13 illustrates some of them, together with their methods of fixing.

Figure 13: Timber-framing anchors
Masonry anchors are used in concrete or masonry. A strong fixing is provided by the casing expanding into the hole as the nut or bolt is tightened. A masonry anchor may be placed into a mortar joint but is far more effective if placed in the body of the masonry. The two most common types are:

Figure 14: Types of masonry anchors
The art of glass-making is very old and, today, the industry still uses basically the same raw materials as the ancient glass makers.
These basic ingredients are:
with the addition of varying quantities of:
The major constituent, silica, is the glass-former while the other minerals act as fluxes and refiners in the melting process.
The raw materials are mixed together and melted at approximately 1500°C and then cooled to a workable temperature of about 1000°C, finally hardening at about 500°C.
Different applications require glass of different thicknesses and properties. The sheet size of the glass area is important: for instance, larger windows require thicker sheets of glass, both for self-support and to resist pressure from wind loads.
Glass is specified by its thickness, method of manufacture and function. Information is readily available from the manufacturers.
Glass used in building falls into the following categories:
Each of the glass types will now be examined.
Floating is the most common modern method for the production of high quality glass for building. It involves a continuous process in which the molten mixture passes to a float bath where it is supported on molten tin. As the ribbon of glass passes through the float bath it is slowly cooled and fed onto rollers.
(refer to Figure 15)

Figure 15: Production of float glass
This is an older method which produces transparent glass that is not perfectly flat. A ribbon of molten glass is drawn between rollers through a cooling chamber (refer to Figure 16).

Figure 16: Production of horizontally drawn sheet glass
This method has been largely superseded by the float glass method. It produces a greater range of thicknesses than the drawn sheet method because the process is continuous. The molten glass is drawn between metal rollers and then between a twin grinder unit which polishes both surfaces simultaneously.
This is produced from ordinary glass by thermal treatment of the finished product. The resultant surface tension across the sheet causes the glass to fracture into small particles when cut so that once the glass product is so treated it cannot be further modified or cut on site. Toughened glass is three to five times stronger than ordinary glass with regard to sustained loads and impact but the surface is no harder than ordinary glass. This type of glass is commonly used for frameless glass assemblies.
This is produced by the addition of certain minerals during melting. It significantly reduces solar heat gain and glare in a building by absorbing between 50 and 90% of the infra-red rays and 30 and 75% of the visible light rays.
As a result, this glass tends to expand and contract more than other types of glass and suitable tolerances must be left in the frame sizes. Heat absorbing glass is available in a small range of tints.
This is produced by coating the glass surface with metallic films. With the use of this glass, solar radiation can be reduced by up to 70%. Frequently this glass forms part of a double-glazing system which protects the coated surface.
This is produced by passing a ribbon of molten glass between rollers during the cooling process so that a pattern is pressed into the glass.
Glass layers are bonded together by heat with a polyvinyl butyric interlayer between the glass layers. This technique produces shatterproof and safety glass such as bullet-proof and cyclone-resistant glass, one-way glass and heat and light reflecting glass.
Wired glass incorporates a layer of the fine wire mesh and is an earlier form of safety glass used for industrial glazing, balustrades, shower screens and so on. It is also used as a fire-retardant glass in some situations.
Glass expands and contracts on heating and cooling and, to prevent the kind of disasters which happened with early glass curtain-walled skyscrapers, this should be taken into account in the design.
Stresses can be set up in the glass resulting from differences in expansion rates between frames and glazing, especially where frames are metal.
Single glazing offers little thermal resistance but the effect of an air gap created by double glazing almost halves the heat loss through a single pane.
The optimum gap is about 20 mm. Heat absorbing and reflecting glasses make an effective contribution to minimising solar heat gain (refer to Figure 17).

Figure 17: Thermal insulation achieved by different glass types
For any degree of sound insulation, double glazing is essential. Sound reduction values vary according to the thickness of the glass and the width of the gap.
Although non-combustible, ordinary glass breaks and then melts in fires and double glazing offers no significant advantage over single glazing. Certain special glasses offer some degree of fire resistance.
Glass fibres are very strong and flexible for their size. They are used in electrical elements and insulators. In addition, their transparent or translucent qualities make them suitable for globes and light shades.
Because of its high electrical resistance, glass is frequently used in electrical elements and insulators. In addition, its transparent or translucent qualities make it suitable for globes and light shades.
Glass bricks can form a semi-transparent wall which is self supporting but not structural. This was a popular building material prior to the 2nd World War, at which time production was interrupted, but it is again becoming popular.
Much glass-making involves the recycling of old glass but glass products have been used in alternative ways as building materials. Glass bottles, for instance, have been built into walls. When filled with water, such walls can act as heat storage banks which can be seasonally adjusted.
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………..
Source: http://bctcwagga.riverinainstitute.wikispaces.net/file/view/Unit+6+Metals+and+glass.doc
Web site to visit: http://bctcwagga.riverinainstitute.wikispaces.net
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes