Polymer: It is an Greek word (poly= many, mares = parts or units)
It is formed by the linking together of a large number of smaller molecules and formed as big molecule is a Polymer. (Each molecule in the polymer is taken as a Monomer)
Ex: Poly Ethylene is formed by linking of large number of Ethylene molecules
n ( CH2─ CH2 ) à ─[CH2─CH2]n─
Types of Polymers:
a) Organic Polymers: The back bone of the polymers made of Carbon atoms.
Ex: Proteins - silk, collagen, and keratin.
Carbohydrates - cellulose, starch, and glycogen, Rubber (hydrocarbon base) and silicones (alternating silicon and oxygen).
b) Inorganic Polymers: The back bone of the polymers made of non Carbon atoms.
Ex: Asbestos
Ex: Fibers, Rubbers, Plastics (Thermo Plastics, Thermo set)
Again synthetic organic polymers classified as 3 types
Ex: poly sulphur nitrides, Poly carborenes
Ex: Silicones
Based on the degree of polymerization these are classified as
Tacticity of Polymers: The arrangement of functional groups on carbon backbone chain of the polymer is called Tacticity of the polymers. They are three types



Mechanism of Polymerization:
Mechanisms are takes place in two ways
1) Step Polymerization 2) Chain Polymerization
1) Step (Condensation) Polymerization: Takes place by the condensation of functional groups of the monomers, hence known as condensation polymerization. These are catalyzed by catalysts. Condensation polymerization is the process of formation of polymers by repeated condensation reactions between two different bi-functional or tri-functional monomers. A small molecule such as water or hydrochloric acid is eliminated in each condensation.
For example: a)Nylon is formed by condensation polymerization of hexamethylenediamine and adipicacid. 
b) Number of molecules of Terepthalic acid reacts with Ethylene glycol and form as Poly Ester

2) Chain (addition) Polymerization: It May be defined as “a reaction occurring between simple polar-group-containing monomers with the formation of polymer and elimination of small molecules like water, HCl, etc.”This reaction yields a polymer product which is the exact multiple of monomers and it is also called as addition polymerization. An initiator is required to start the reaction. It proceeds through three steps (Initiation, propagation & termination).
Ex: a) n (ethylene)![]() --------à polyethylene
--------à polyethylene ![]()
b) n( vinyl chloride) ![]() -------à poly vinyl chloride (PVC)
-------à poly vinyl chloride (PVC) 
c) n (propylene)  ---------à polypropylene
---------à polypropylene 
These are four types
• Radical Polymerization: The initiator is a radical, and the propagating site of reactivity is a carbon radical.
• Cationic Polymerization: The initiator is an acid, and the propagating site of reactivity is a carbocation.
• Anionic Polymerization : The initiator is a nucleophile, and the propagating site of reactivity is a carbanion.
• Coordination Catalytic Polymerization: The initiator is a transition metal complex, and the propagating site of reactivity is a terminal catalytic complex.
Mechanisms: Polymerization of a monomer (ethene…) to polymer (polythene) consists of heating or exposing to light a mixture of monomer (ethene) with a small amount of benzoyl peroxide as the initiator
I ― I ---------à 2 I* (radical)
I (radical) + CH2 = CH─X à I─CH2─CHX (radical)
b) Propagation: The monomer radical reacts with number of monomers and form as a long chain called living polymer.
I─CH2-CH*X (radical) + CH2 = CH─X à I─CH2-CHX ─CH2─CH*X
I─CH2-CHX ─CH2─CH*X + n(CH2 = CH─X) à I─CH2-CHX ─(CH2─CHX)n─ CH2─CH*X (living polymer)
c) Termination: By coupling of two living polymers form a dead polymer.
I─CH2-CHX─ [CH2-CHX]n─CH2─CH*X
+ à I─CH2-CHX─ [CH2-CHX]n─CH2 = CHX
I─CH2-CHX─[CH2-CHX]n─CH2─CH*X
BF3 + H2O à [F3BOH]- H+ (initiator)
[F3BOH]- H+ + CH2 = CHX ---------à CH3─C+HX [HOBF3]--
CH3─C+HX [HOBF3] -- + CH2==CHX -----àCH3─CHX─CH2 C+HX [HOBF3]-
CH3─CHX─CH2 C+HX [HOBF3] -- + n CH2 ==CHX ----à CH3-CHX─ [CH2-CHX] n-- CH2 C+HX [HOBF3]—
c) Termination: The above polymer coupling with OH—ion and terminates thr reaction.
CH3-CHX─[CH2-CHX]n- CH2 C+HX[ HOBF3]— -à CH3-CHX─[CH2-CHX]n-- CH2 CHOH + BF3
a) Initiation: The initiator reacts with monomer and forms a monomer carbanion
Na+R-- + CH2==CHX à Na+ + R—CH2—C–HX
b) Propagation: The monomer carbanion attacks the pi electrons of the other monomers and forming as a long chain (living polymer).
R—CH2—C—HX + n (CH2 ==CHX) à R-- CH2-CHX─ [CH2-CHX] n─CH2-- C—HX
c) Termination: The above polymer coupling with HCl and terminates the reaction.
R-- CH2-CHX─ [CH2-CHX] n─CH2-- C—HX + H+ Cl- à R-- CH2-CHX─ [CH2-CHX] n─CH2—CXH2
4) Co-ordination / Zeigler-Natta polymerization: Zeigler-Natta discovered a catalyst called Zeigler – Natta Catalyst. It is a combination of transition metal halides (TiCl4 / ZnBr3) with organometallic compounds like (C2H5)3Al. some polymerizations can be carried out by this catalyst is called as Zeigler-Natta polymerization (Catalysis) reaction.
a) Initiation: The catalyst reacts with monomer and forms a monomer catalyst complex
Cat—R + CH2==CHX à Cat—CH2—CHRX
Cat—CH2—CHRX + n (CH2==CHX) à Cat-- CH2-CHX─ [CH2-CHX]n─CH2—CHXR
Cat-- CH2-CHX─ [CH2-CHX]n─CH2—CHXR + HX àCH3-CHX─[CH2-CHX]n─CH2—CHXR
S.No |
General formula |
X =? |
Molecule Name |
Polymer Name |
1 |
CH2=CH-X |
H |
Ethylene |
|
2 |
CH2=CH-X |
Cl |
Vinyl Chloride |
|
3 |
CH2=CH-X |
C6H5 |
Styrene |
|
4 |
CH2=CH-X |
CH3 |
Propylene |
|
5 |
CH2=CH-X |
CN |
Acrylo Nitrile |
|
6 |
CH2=CH-X |
O-CO-CH3 |
Vinyl Acetate |
|
7 |
CH2=CH-X |
CO-O-CH3 |
Methyl methacrylate |
|
Plastics:
These are the polymers characterized by the property of the permanent deformation in structure on applying some stress/force, moulded to desired form subjected to heat and pressure in the presence of catalysts. Resin is the product of polymerization and forms the major part of the plastics. These are light weight, good thermal & electrical insulators etc.,
These are classified as two types
a) Thermo Plastics: These resins are become soft on heating & rigid on cooling reversibly. They have three dimensional network structures. The cross links and bonds retain their strength on heating and hence they do not soften on heating.
Ex: polyethylene, PVC etc.,
b) Thermo sets: These resins are moulded; once they are solidified they cannot be softened. Ex: Bakelite, Nylon etc.,
Differences between Thermo Plastic & Thermo Set Plastics
S.No |
Thermo Plastic |
ThermoSet |
1 |
These becomes soft on heating & Becomes |
Once these are solidified they cannot be softened by heat |
2 |
thermoplastics can be remelted and |
they cannot be recycled |
3 |
They formed by chain polymerization |
They formed by step polymerization |
4 |
These are soft, weak and less brittle |
These are hard, strong and more brittle |
Compounding & Fabrications of Plastics:
Compounding: The polymer resin is mixed with 4 to 10 ingredients during fabrication to impart in to different shapes. The ingredients are
a) Resins: 1) the product of polymerization and forms the major part of the plastics
2) It acts as a binder which holds all the ingredients together.
Ex: linear chain polymers are converted to cross-linked polymers.
b) Plasticizers:Plasticizers are substances added to enhance the plasticity of the material and to reduce the cracking on the surface. Plasticizers are added to the plastics to increase flexibility and toughness. Plasticizers also increase the flow property of the plastics. These increase the flexibility of the polymer. These decrease the intermolecular forces of attraction between polymer chains. Ex: Camphor, vegetable oils, Castor oil etc.,
c)Fillers:give better hardness ,strength to the plastics & These reduce the cost of the polymers. Fillers are generally added to thermosetting plastics to increase elasticity and crack resistance. Fillers improve thermal stability, strength, non combustibility, water resistance, electrical insulation properties and external appearance.
Example wood flour, Asbestos, Mica, Cotton, Carbon black, Graphite, Barium sulphate etc.
d) Lubricants: They make moulding of plastic easier & gives non sticky to the moulds. Ex: Waxes, oils….
e) Catalysts : They accelerates the polymerization during the moulding operation ex: benzoyl peroxide……….
f) Stabilizers: they improve thermal stability during polymerization.
Ex; White lead, red lead ……
Fabrications:
This method is applied both for thermoplastic and thermoset. Solid plastic material is filled in between the cavities which are movable to each other. When heat and pressure are applied the cavities filled with fluidized plastic. The two moulds are closed to each other and curing (time taken for setting or cooling) is done either by heating incase of thermo plastics or by cooling incase of thermo set. After curing the moulded material is taken out by opening the mould. 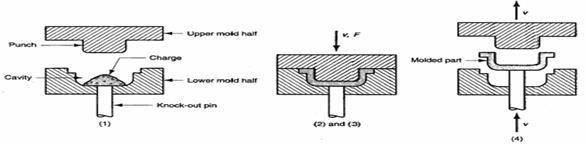
This is used for the fabrication of thermoplastic resins. The compound plastic is taken from the hopper from they move through a tube having proper dimension for the accurate quantities. The plunger moves to and fro in the cylinder to send the plastic into the spreader in a heating chamber where the materials are converted to viscous liquid push through a nozzle into the mould maintain at room temperature. The molten material sets in the mould which is sometimes water cooled and pressure is maintained. After curing the moulded material is taken out by opening the mould.

The powdered compounded plastic material is in a chamber applied with minimum temperature and high pressure till it begins to become soft and semisolid. Then it is injected into the mould by a plunger working at high pressure .the soft plastic material is put into the mould which is heated up to the curing* temperature.

It is used for moulding of thermoplastic materials into articles of uniform cross section like tubes, rods, sheets, wires cables etc. the thermoplastic materials are heated to semi solid state and then pushed by means of a screw conveyor into a die, having the required outer shape of the article to be fabricated. The extruded article gets cooled due to atmospheric exposure or artificially by air jets, by water sprayer in a long conveyor which carries away the cooled product. 
Blowing: It is also known as bubble casting is used for fabricating hallow plastic materials like soft drinking bottles, containers etc.
A hot, softened thermo plastic tube called parison is placed inside a two piece hallow tube in the mould. The halves of the mould are closed & parison is now blown by blowing compressed air through the blowing pin. the hot parison is inflated like a balloon & goes on expanding until it comes in intimate contact with the relatively cold interior surface of the hallow mould & assumes the shape of the hollow cavity. The moulded material is taken out by opening the mould.

Polymers of thermo plastics and thermo set resins:
Ethylene is prepared by the hydrogenation of acetylene
C2H2 à CH2=CH2
a) By using Free radical polymerization mechanism, low density polyethylene is prepared at 80ºc.
n ( CH2=CH2 ) ---------------à ![]()
Benzoyl peroxide
b) By using coordination polymerization mechanism, High density polyethylene is prepared at 120ºc.
This can be obtained by the polymerization of ethylene at 1500 atm and a temperature 150 – 250 0C in presence of traces of oxygen.
Properties:
Depending upon the density, they may be LDPE and HDPE. If we use free radical initiator, LDPE is the product while use of ionic catalysts results in the formation of HDPE.
It is a rigid, waxy white solid. Translucent. It is permeable to many organic solvents. It crystallizes easily.
LDPE has a density 0.91 to 0.925 g/cm3 HDPE has a density 0.941 to 0.965 g/cm3
HDPE is linear and has better chemical resistance.
Uses: These are useful in the preparation of insulator parts, bottle caps, flexible bottles, pipes etc.
LDPE is used in making film and sheeting. Pipes made of LDPE are used for both agricultural, irrigation and domestic water line connections.
HDPE is used in manufacture of toys and other household articles.
Properties: a) It is rigid, waxy white, translucent, non polar, with high symmetrical structure
Engineering Applications: It is used for making high frequency insulators, bottle caps, tubes, coated wirers, kitchen and domestic appliances.
2. PVC: ![]()
Vinyl chloride is prepared by hydrohalogenation of acetylene.
![]() CH = CH + HCl CH2 = CH Cl
CH = CH + HCl CH2 = CH Cl

Poly Vinyl Chloride is obtained by heating a water emulsion of vinyl chloride in presence of a small amount of benzoyl peroxide or hydrogen peroxide in an auto clave under pressure.
Vinyl chloride, so needed is generally prepared by treating acetylene at 1 to 1.5 atmospheres with hydrogen chloride at 600C to 800C in the presence of metal chloride as catalyst.
Properties: It occurs as a colourless rigid material.
It is having high density and low softening point.
It is resistant to light, atmospheric oxygen, inorganic acids and alkalis.
It is most widely used synthetic plastic.
Uses: It is mainly used as cable insulation, leather cloth, packing and toys.
It is used for manufacturing of film, sheet and floor covering.
PVC pipes are used for carrying corrosive chemicals in petrochemical factories
Engineering Applications:
3)Ploy styrene: It is prepared when ethylene reacts with Benzene in the presence of AlCl3.
CH2=CH2 + C6H6 ----------à C6H5-CH=CH2
It is prepared by free radical polymerization, in the presence of benzoylperoxide

Properties: a) It is stable to light & moisture.
Engineering Applications : It is used for moulding of radio & television parts, refrigetor parts, battery cases, lenses, indoor lighting panels etc.,
4) Bakelite:
It is prepared by the step polymerization reaction of phenol with formaldehyde in presence of an acid. The polymerization is

Properties:
1) It is hard, rigid, and strong polymer.
2) It is a scratch resistant and water resistant polymer
3) It is a good anion exchanging, electrical insulator, resistant to atmospheric conditions like O2, CO2 etc
Applications:
RUBBER
Natural rubber: Rubbers also known as Elastomers, they are high polymers, which have elastic properties in excess of 300%.
Natural rubbers consist of basic material latex, which is a dispersion of isoprene. During the treatment, these isoprene molecules polymerize to form, long-coiled chains of cis-polyisoprene. Natural rubber is made from the saps of a wide range of plants like Hevea brasillians and guayule.
Latex: is a milky white fluid that oozes out from the plant Hevea brasillians when a cut is made on the steam of the plant.
The latex is diluted with water. Then acetic or formic acid is added [1kg of acid per 200kgs of latex] to prepare coagulum. This is processed to give wither crepe rubber or smoked rubber.
Vulcanization:
Vulcanization discovered by Charles Goodyear in 1839.
It consists of heating the raw rubber at 100 – 1400C with sulphur. The combine chemically at the double bonds of different rubber spring and provides cross-linking between the chains. This cross-linking during vulcanization brings about a stiffening of the rubber by anchoring and consequently preventing intermolecular movement of rubber springs. The amount of sulphur added determines the extent of stiffness of vulcanized rubber. For example, ordinary rubber (say for battery case) may contain as much as 30% sulphur.
Advantages of vulcanization:
By passing the compounds through calendaring chamber we can get a rubber sheet of uniform thickness.
Synthetic Rubbers or Elastomers :
They are characterized by the property of elasticity. Some of the important Elastomers are given below
1) BUNA-S : It is also called as Styrene rubber or GRS ( Government Rubber Styrene) or Ameripol. It is the composition of the monomers & catalyst.
BU - stands for Butadiene – monomer
NA - stands for sodium – catalyst
S - stands for styrene – monomer
BUNA-S is produced by the copolymerization of butadiene with styrene using sodium as a catalyst.

The monomers butadiene & styrene were synthesized in the following way
Butadiene is produced from acetaldehyde & ethyl alcohol as
CH3CHO + CH3-CH2-OH à CH2 = CH – CH = CH2 + 2 H2O
Styrene is from Benzene & ethylene in the presence of anhydrous aluminium chloride catalyst.
CH2 = CH2 + C6 H6 à 
Properties:
a) It is a strong & tough polymer.
b) It posses excellent abrasion resistance.
c) Styrene rubber posses high load bearing capacity & resilience.
d) It is a good electrical insulator.
Applications: a) major application of styrene rubber is in the manufacture of tyres.
b) It is used in the footwear industry for making shoe & footwear components.
c) It is also used for making wires & cable insulators.
d) Used for the production of floor files, tank linings in chemical industries & as an adhesives.
2) BUNA-N : It is also called as Nitrile Rubber or GRA ( Government Rubber Acrylo Nitrile) .
Its composition is BU stands for butadiene – monomer
NA stands for sodium - catalyst
N stands for Acrylo Nitrile – monomer
Butadiene is produced from acetaldehyde & ethyl alcohol as
CH3CHO + CH3-CH2-OH à CH2 = CH – CH = CH2 + 2 H2O
Acrylo Nitrile is prepared by the addition of hydrogen cyanide to acetylene.
C2 H2 + HCN à CH2 = CH.CN
Properties: a) It posses excellent resistant to heat, sunlight, oils, acids, salts & less resistant to alkalis than natural rubber.
b) It is astrong & tough polymer with light weight.
c) It is also vulcanized with sulphar
d) Resistant to atmospheric conditions like O2, CO2 and moisture.
Applications:
a) Used for making conveyor belts, high altitude aircraft components & automobile parts
b) Used for making tank linings & pipes for chemical industries
3) Butyl Rubber:
It is also known as GR-I ( Government rubber isobutene), is produced by the copolymerization of isobutene with 1-5% of butadiene , in the presence of anhydrous AlCl3 in methyl chloride as a catalyst.
n( CH2 = CH(CH3)2 ) + n ( CH2 = C.CH3 ─CH = CH2 )

Properties:
a) It’s a strong & tough rubber with low permeability to air & other gases.
b) It posses excellent resistant to heat, abrasion, ageing & chemicals.
c) It is a good electrical insulator.
d) It is also vulcanized with sulphar
Applications:
a) Used for making cycle tyres & automobiles.
b) Used for making automobile parts, conveyor belts in food processing industry
c) Used as an insulator for high voltage wires & cables.
4) Thiokol Rubber: It is prepared by the copolymerization of ethylene dichloride & sodium disulphide.

Properties: a) It posses high strength & impermeability to gases.
b)It cannot be vulcanized & it can not form Hard rubber.
c) It posses excellent resistant to mineral oils, fuels, oxygen, ozone & sunlight.
Applications: a) It is used in making Gaskets & seals for printing rolls.
b) Fabrics coated with Thiokol are used for barrage balloons, life rafts & jackets which are inflated by CO2.
5) Polyurethane:
The copolymerization of ethylene diisocyanate & ethylene glycol produces polyurethane by poly addition mechanism.

Properties: a) These are highly resistant to oxidation because of their saturated character.
b) It is a good electrical & thermal insulator.
c) They show good resistance to many organic solvents & oils but attacked by acids, alkalis especially concentrated & hot.
Applications: a) It is used for surface coatings.
b) Its major utility is as a thermal insulator for refrigerators etc.
6) Silicone Rubber:
By the hydrolysis of Dichloro silanes followed by condensation polymerization of dialkyl silanes yields silicone rubber.
![]()
Properties: a) They posses strength less than Natural rubber.
b) It is a good thermal insulator & resistant to extreme temperatures
c) It posses good dielectric strength.
Applications: a) It finds extensive uses as sealing materials, gaskets, diaphrams & rollers.
b) These are extensively used in air crafts & automobile industry.
c) Domestic oven doors are lined with silicone rubber.
The polymers which can be drawn in the form of long filaments with high tensile strength, high stiffness & irreversible deformation are called Fibers.
These posses high crystallisability & high melting points. Fibers are classified depending on their origin into
1. Natural fibers (Cotton, Wool, Silk …..)
2. Synthetic fibers (Polyester, Nylon…)
Fiber reinforced Plastic:
It is produced by reinforcing a plastic matrix with high strength fibers such as glass, graphite, alumina, carbon, boron.
Natural fibers: Sisal, Asbestos…..
Depending on the desired properties of final reinforced composite, the nature of the fiber used Is decided.
Glass fiber is the most extensively used reinforced because of durability, acid proof, water proof & fire proof nature of glass. Glass is drawn in the form of long filaments fire than cotton or silk thread. Then the filaments are woven in the form of mats. The fiber material is suitably bonded with plastic materials to be reinforced.
The common plastics are Polyesters, epoxy, silicone, melamine, vinyl derivatives & polyamides.
The following are the various processing techniques
1) Matched metal die Moulding: This is pres moulding method under a temperature of
235-2600F & 200 – 300 psi pressure. The upper mould containing resin & reinforcing fibers is pressed on to the lower mould. It is most efficient & economical method.
2) Injection Moulding: This method is used for reinforced thermoplastics. A mxture of fiber & resin are allowed through a nozzle into the heated mould & then cool.
3) Hand – lay –up: The reinforcing mat fiber is cut to fit in a mould & saturated with resin by hand brush. Layer built up gives the thickness of the article.
4) Spray –up: Small amount of reinforcement & resin are deposited on the mould by using a specially designed spray gun. cooling(curing) takes place at room temperature.
5) Continuous Lamination: fabric & resin are run through laminating rollers between cellophane sheets to control the thickness & resin continent. They are then cured in heating chamber.
Advantages of FRP: a) Low efficient of thermal expansion.
b) High dimensional stability.
c) Low cost of production.
d) Good tensile strength.
e) Low dielectric constant.
Biodegradable Polymers: These are defined as a Polymer in which degradation results from the action of naturally occurring micro organisms such as bacteria, fungi & algae.
The biodegradable polymers may be naturally occurring OR they may be synthesized by chemicals.
* Naturally occurring Biodegradable Polymers: These are classified in to four groups as
1) Poly saccharides: Starch & Cellulose
2) Proteins: Gelatin, Casein, silk, wool.
3) Polyesters: polyhydroxy alkanolis
4) Others: Lignin, Shellac, Natural rubber
The rate of degradation & the formation of metabolites depend very much as the structural complexity of the material & the environmental conditions selected for degradation.
* Synthesized biodegradable Polymers: These are derived from petrochemical or biological sources that are biodegradable. Many of these polymers are considered as special materials for example dissolving suture material used in medical field, biopolysters. Because of their high prices these polymers could not gain popularity, later a sustainable interest was created in the devolpement of chemicals derived from forming of particular crops.
Synthetic resins are a) Polyalkylene esters b) Polyacetic acid c) Polyamide esters
d) Poly Vinyl esters e) Polyvinyl alcohol f) Poly anhydrides
Applications: a) the compostable bags help in the disposal of the vegetable mater being converted to CO2 & CH4.
b) The Problem of land fills by solid waste can be reduced.
c) Several test methods are developed to determine the compo stability of environmentally degradable plastics.
Conducting polymers:
Those polymers conduct electricity are called as conducting polymers, is due to the presence of unstauration or due to externally added ingredients in them. Depending on this they are different types
Intrinsic polymers :
Conduction due to intensive conjugation of double bonds in their structure i.e. back bone of the polymers. Again these are two types
Polymers having conjugated double bonds in the back bone posses their conductivity due to ∏ electrons. In ∏ bonding the overlapping of the orbital’s is lateral over the entire back bone resulting in the formation of valence bands & conducting bands which were separated by a significant Fermi energy gap. By applying temperature the electrons jump the gap & reach into conductance band.
1) C2H2 à

2) Formation of polyaniline

b) Doped conducting polymers:
The conducting polymers having ∏ electrons in the backbone can easily oxidize or reduced because they posses low ionization & high electron affinity. Hence their conductivity increases by the addition of positive charge or negative charge by oxidation or reduction is called Doping. It is two types a)Creating a positive site on polymer is called P-doping.
b) Creating a Negative site on polymer is called N-doping.
P-doping:
It is conducting by the oxidation of polymer like poly acetylene with Lewis acid or iodine vapors
― [CH = CH]n― + 2 FeCl3 à ―[CH = CH]n+― FeCl4─ + FeCl2
― [CH = CH]n― + 3 I2 à 2 ―[CH = CH]n+― I3─
During oxidation the removal of ∏ electrons lead to the formation of a delocalized radical ion called polaron having a hole in between valance band & conducting band.

Polyacetylene forms positively charged solutions (p-type doping) due to its degenerate ground state structure.

The second oxidation of the polaron in two positive charge carriers in each chain called bipolaron. These carriers responsible for conductance when placed in electric field.
N-doping:
It is carried out by reduction of polymers by using reducing reagents like sodium napthalide (Na+ (C10H8)-) .Bipolar on takes place in two steps, followed by recombination of radicals which yields two charge carriers on the Polyacetylene chain. 
The electron added to polyacetylene by reducing doping does not go into the conductance band but goes in to an intermediate electronic state with in the band gap of radical anion.
Extrinsically conductance polymers
The conductivity of the polymers due to the presence of externally added ingredients in them. These are classified as two types.
a)Conducting element filled polymers:
The polymer acting as a binder to hold the conducting element such as carbon black, metallic fibers etc. Minimum concentration of conducting filler (percolation threshold) is added so that the polymer starts conducting. The most preferred filler is Carbon black has high surface area, more porosity etc.,
b)Blended Conducting polymers:
The polymer is blended with a conducting polymer to improve physical, chemical, electrical & Mechanical properties.
Ex: 40% poly parole is conventional polymer give higher impact strength. These blended polymers are used in electromagnetic shielding.
Liquid crystal Polymers ( LCP )
These are the materials that behave in some ways solid & in some ways like liquid.
Stephanie kwolek had made the polymer liquid crystal solution based on the polyamide named Kevlar & its monomer unit is

When this polymer dissolved in tetra methyl urea & CaCl2, the polymer molecule behaves strongly. It is long, straight, stiff, lined up like logs floating down a river, because of the strange opascent look of the solution.Molecules of solid materials are arranged in order is called as Crystals.
Since the Kevlar structure is a liquid, but its molecules are arranged, the solution is called a Liquid crystal. These can be classified into two types
a) Liquid crystallinity in polymers may occur by dissolving a polymer in a solvent, which are called lyotropic liquid crystal polymers. Eg: Kevlar
b) Liquid crystallinity in polymers may occur by heating a polymer above its glass transition temperature or melting point, which are called Thermo tropic liquid crystal polymers.
Eg: Vectra

Properties:
a) These have High mechanical strength at high temperature.
b) These posses extreme chemical resistance
c) They posses inherent flame retardancy & goof weather ability
d) LCP can be welded. The lines created by welding are the weak ponts in the resulting products.
e) LCP resist stress cracking the presence of most chemicals at elevated temperature.
Applications : a) LCP are some times called super polymers. Their wide range of exceptional properties & case of processing make them design for many demanding applications.
b) The electrical motor components are made from LCP.
c)LCP finds extensive applications as coatings, composites & additives
d) LCP finds its applications in electronic industry as LED’s & SMT components.
Source: http://www.chemistry4all.yolasite.com/resources/Polymer.doc
Web site to visit: http://www.chemistry4all.yolasite.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes