Solders applied with a soldering iron are used for fastening many types of sheet metals and for making seams watertight.
To achieve a good standard of soldering, you must first know the kind of material being soldered, the kind of solder and flux most suited and the proper soldering bit for the job.
The term solder is a broad one, but it generally refers to the joining or uniting of two or more pieces of metal by means of an alloy having a lower melting point than that of the metal being soldered. Soldering may be divided into two general classes, soft and hard soldering.
Hard solders are those include such processes soft soldering that is having a melting point over 750°F and include such processes as silver soldering and brazing. It is soft soldering that is generally used by the sheet metal worker.
Soft solder comprises of tin and lead and its melting point depends largely upon the proportions of these two elements. The common soft solders are divided broadly into three grades of the following approximate compositions -
TIN LEAD
(1) Best tinman’s solder 2 1
(2) Medium quality 1 1
(3) Plumbers solder 1 2
Soft solders are classified by the proportions of each element by weight. Therefore 50-50 means that the solder consists of 50% tin and 50% lead by weight. It is important to note that the % of tin is always given first. Thus 60-40 contains 60% tin and 40% lead while 40 - 60 contains 40% tin and 60% lead.
In the sheet metal shop, the preferred solder proportions are 50-50 since this gives a low melting point. Other trades have found that different proportions are better suited to their needs. Generally by increasing the tin % it lowers the melting point, this is only true up to 87% tin content, after this point as the tin % increases, the solder’s melting point increases.
It is well to remember that the strength or bonding quality depends on more than the melting point of the solder. For example, a poorly tinned soldering iron may be overheated to 700°F and still not distribute the solder in a satisfactory way.
In the sheet metal trade, solder is generally used in bar or wire form. In wire form it can either be of hollow or solid cross section. In the hollow form, the core can contain an acid and rosin flux. This is called acid core and rosin core solder.
Flux is required to remove the oxide film that is always present on metal. If no flux were used, this oxide film would make adherence of the solder to the metal difficult if not impossible. Rather than bonding the metal, the solder would lie loosely over the oxide film.
There are two general classes of soldering flux - corrosive, and non-corrosive. Acids are corrosive and therefore should be washed off immediately after the soldering has been completed. Rosin is a non-corrosive flux. It may be in the form of a lump, powder, paste or liquid.
Rosin is a by-product of oil and of turpentine and is a common, non-corrosive type of flux for soldering metals such as bright tin plate, terne plate and copper. Rosin may be applied as a powder melted on the metal by a warm soldering iron, or made into a paste by adding enough benzine to make it a semi-solid.
Muriatic acid is the commercial grade of hydrochloric acid used in soldering. It is used for making zinc chloride for a flux on galvanised steel and for cleaning off dirty parts of the metal before they are soldered. In the sheet metal shop, muriatic acid is usually called "raw spirits". Though the term hydrochloric acid and muriatic acid are often used inter-changeably, there is one difference of which you should be aware. Muriatic acid is the commercial term and comes in one strength only - a medium strength acid.
Hydrochloric acid is the chemical name and can be in any strength from a very dilute to a very concentrated and dangerous acid.
Raw hydrochloric acid is colourless and has a sharp odour. However, the surest identification of raw acid is to put a small amount on a piece of galvanised sheet. If the metal bubbles and turns black it is raw acid.
Zinc chloride is often called "killed spirits". It is unequal as a flux when soldering galvanised metals, zinc, brass, copper, lead. It is also used to solder tin that has become tarnished by being exposed to the weather. "Killed spirits" is colourless and odourless. It is as corrosive and dangerous as raw acid and it should be treated with the same respect.
One method of distinguishing "killed spirits" from raw acid is to put it on a piece of galvanised sheet. If it reacts as before it is said to be raw acid, if no reaction takes place it is "killed spirits".
With both "killed spirits" and raw acid it is extremely important to remember that these acids are highly dangerous and should be treated with the utmost caution. They will eat holes in clothing and cause painful skin burns.
The greatest danger is if it comes into contact with the eyes.
Should this happen, wash the eyes out with cold water and send for a doctor.
Soldering irons are made of solid copper pieces and vary in shape and weight. The body of the soldering iron is called the head.
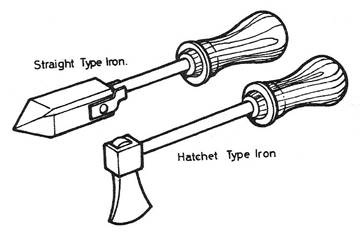
Figure 3 - Soldering Irons
The pointed end is referred to as the point or tip and is forged into various shapes for different kinds of work. Common shapes would include the square pointed or straight type, and the hatchet type. The weights of soldering irons vary depending on the job being done, but a standard weight would be approximately ¾ lb to 1½ lbs.
The handles are made of wood or fibre. A good handle would have a metal ferrule to keep it from splitting and a cheap one would have a ferrule wire to prevent it from splitting.
Figure 4 - Soldering Iron Handles
A special handle flange for heavy irons would have a flange to keep the heat away from the handle.
Sheet metal workers generally use a bench gas furnace or a butane furnace.
The bench type gas furnace is specially designed to heat soldering irons.
Figure 5 - Gas Furnace
The furnace is equipped with shut off valves and a pilot light. The base and the cover of the furnace are lined with asbestos. A steel shelf is located in the rear to protect the tinned points of the iron. For safety every furnace should be in a well ventilated area. It is preferable that some type of extraction system be fitted to extract gases etc.
When lighting observe the following:
When not in use, the furnace burner valve should be turned off, leaving only the pilot light burning. At the close of the day the main line valve and the branch line valve should be turned off.
The butane furnace is often used in the shop. It is portable and safe to use, providing you follow the manufacturer's instructions.
Figure 6 - Butane Furnace
Tinning a soldering iron means covering the point with solder. A well tinned iron is absolutely necessary to do a good job.
There are several important reasons for tinning a soldering iron.
Before tinning the iron you will heat it to a cherry red then file it to remove any pits or scale, then reheat it and then apply a small amount of flux and solder to the point, it will flow smoothly if the point is clean and hot enough.
If it does not adhere to it you will have to reheat the iron and clean and proceed again.
The soldering iron should not be overheated because the tinned surface will be ruined no matter how well it’s tinned. When not in use the heat should be lowered and the iron withdrawn from the heat.
It is very important to apply the flux correctly. Improper use has destroyed many good jobs. Liquid fluxes are applied by brush, taking care not to drop any except where it's to be soldered.
Rosin may be either sprinkled on, or be melted on the job by a hot iron.
The positioning of the soldering iron is important because the iron does two things as it is applied to the parent metal.
When soldering galvanised steel the principal question which arises is, whether you should use raw acid or zinc chloride as the flux. If the metal is bright and clear, zinc chloride can be used to make a well-bonded joint. However, if the metal is dull and dirty, raw acid definitely should be used. There are also good commercial marketed fluxes for soldering galvanised steel.
Steel, coated with tin or lead, can be soldered with a variety of fluxes. Whenever possible, rosin should be used because of its non-corrosive action upon metal. If the metal is dull, as would be true of used or old tin plate, zinc chloride can be used satisfactorily. Always wipe or wash the flux from the metal immediately after completing the soldering.
If the surface of the copper or brass is free from oxide it can be soldered easily using zinc chloride as the flux. If the copper or brass is dull, it can be cleaned by using raw acid directly on the metal with a brush. The raw acid should be washed off with a damp rag before applying the zinc chloride for soldering. All flux should be washed off after soldering.
It can be soldered easily with prepared soldering fluxes designed especially for the purpose. If these special fluxes are not available, prepare the surface in the same way as that of tarnished copper. Scratch the surface with sandpaper and with a brush apply raw acid to the soldering parts. After the acid has been left on the metal for the required time (which varies with the type of stainless steel and the acid strength) it should be wiped clean with a damp rag. The metal is then soldered as for copper, using regular half and half solder with zinc chloride as the flux. The flux should be cleaned off after soldering.
Rosin flux is considered best for soldering lead and the surface must be scraped with a tinners scraper immediately after cleaning as the metal quickly oxidises. The soldering iron must be well tinned and cooler than usual. There should be some solder between the hot iron and the lead at all times to keep the lead from melting.
A greater rate of heat flow can be accomplished in some soft-soldering operations by the use of a suitable flame. A mouth 'blow-pipe' may be used where soldered joints are required to be made with a minimum of heating of the article as a whole. By this means a bunsen-type flame of exactly the right size can be directed where the heat is required, solder being applied when the locally heated part has reached soldering temperature. Special 'blow-pipe solder' is available for the purpose, or solder can be applied from fine flux-cored wire or in the form of a paste in which the flux and solder are incorporated.

A small soldering bit is not suitable for soldering any heavy or comparatively large-size sheet-metal products.
Reason: When the copper bit is too small for the job, it cannot maintain an adequate temperature to allow the solder to flow freely and to penetrate into the seam or joint.
When an inadequate size of copper bit is heated to the correct temperature and applied to thick gauge sheet metal, the small amount of heat stored in it is rapidly conducted away by contact with the relatively large mass of cold metal.
Result: The small copper bit cools very quickly causing the molten solder to chill and render the joint ineffective.
When using a small copper bit for the soldering of small parts, it is very important not to rest the parts on a cold mass of metal. Where possible small parts to be soldered should be places on some sort of insulating material such as a block of wood. Similarly when parts to be soldered are to be held in a vice, wood or fibre faced clamps should be used.
(a)Lack of heat when using too small a soldering iron

The use of a large size copper bit provides a much greater heat source.
A large reservoir of heat will compensate any heat loss by conduction when the bit makes contact with a relatively cold mass of metal.
The tinned facets of the copper bit actually making contact with the surface of the metal to be soldered should be of sufficiently large area.
Reason: A large area, tinned face on the copper bit will provide a much greater heat concentration. A large concentration of heat will allow time for it to flow into the work while the bit is slowly drawn along the surface of the metal.
Preheating parts prior to soldering will reduce heat loss by conduction.
(b) Adequate heat concentration is provided by the use of a large enough soldering iron.

Large gas flames or 'blow-pipes' are extensively used where a more rapid and wider spread of heat is required, for example:

Figure 7 - Flame Soldering
Soft soldering, as a joining method, relies almost entirely on ADHESION for its strength.
Soft-soldered joints are strongest at room temperature, their mechanical strength decreasing rapidly as the temperature increases.
The strength of a soft-soldered joint is determined by three basic factors:
Factors influencing joint design for soft soldering are illustrated in Figure 8 and Figure 9.
As illustrated in Figure 8(a), a soldered joint is strongest 'in shear' (42-49 MN/m²) and weakest 'in tension' (0.28-0.35 MN/m²).
A straight butt joint under tension places the bulk solder between the interfaces under TENSILE STRESS (see Figure 8(b)). This means that the stress-carrying capacity of this type of joint is limited to the tensile strength and cross-sectional area of the solder itself. Soft solder has a very low tensile strength.
Joints designed for 'shear loading' can be strengthened by increasing the lap area (surface contact area) as required, and by reducing the thickness of the solder deposit (Figure 8(e)).
Soldered lap joints made with 'close clearances' derive the greater proportion of their overall strength from the 'bonded zones' (interfaces) where the solder is in intimate contact with the surfaces of the parent metal. Any tendency to yield under load is opposed by the force required to displace the solder bond.

Figure 8 - Design of Soldered Joints
Figure 10, Figure 11 and Figure 12 illustrate three common soft-soldering operations: tacking, sweating and floating.
Tacking (Figure 10). When making long soldered seams it is considered good practice to “tack” the surfaces together using the following sequence.
Figure 9 - Various Types of Soft-Soldered Joints
First at the middle, then at each end, and at regular intervals in between.
Reason: This tacking sequence maintains a uniform spread of temperature and so reduces any tendency by the parent metal being tacked to warp or buckle due to uneven expansion and contraction.
Figure 9 shows various types of joint design used in sheet-metal work.
The process of tacking (Figure 10(b)) is simply carried out by methodically placing small ‘blobs’ of solder at regular intervals along the seam in order to hold it in position prior to the main soldering operation.
The tip of the hot copper bit is used and the local heating effect causes the small amount of solder placed on it to penetrate the joint at the point of contact.
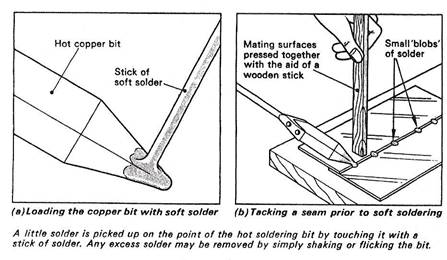
Figure 10 - Soft-Soldering Processes – Tacking
During tacking, the mating surfaces of the seam are pressed tightly together by means of a wooden stick or the 'tang' of a file.
The second common soft-soldering process is illustrated in Figure 11(a), (b) and (c).
Figure 11 - Soft-Soldering Processes – Sweating
Figure 12 - Soft Soldering Processes – Floating
THE SUCCESS OF SWEATING DEPENDS UPON HAVING A CONSTANT SUPPLY OF HEAT.
The soldering bit must be large enough to transmit sufficient heat through the joint.
FLOATING, the third common process and illustrated in Figure 12, is a useful soft-soldering operation which is employed when sealing SELF-SECURED JOINTS in order to make vessels or containers liquid-tight.
One method, illustrated in (a), is to use a specially shaped soldering bit called a BOTTOMING IRON.
The joint is fluxed both inside and outside, and the container is supported on a wooden base and tilted at an angle of approximately 45°.
A small quantity of soft solder, conveniently prepared in the form 'buttons' or 'blobs', are dropped in the container and melted with the heated and tinned bottoming iron.
This molten solder is then carefully flooded along the inside of the joint as the container is slowly rotated. The quantity of solder is uniformly controlled by steady manipulation of the soldering iron, adding blobs of solder if and when required.
If a bottoming iron is not available, an alternative method is to use a flame as the heat source. This method is shown in Figure 12(b).
An asbestos glove should be worn as a protection from the flame and the heat radiated from it. The flame is carefully applied to the outside of the joint, and the same procedure is adopted.
With either method the solder not only penetrates the interlocking surfaces but presents a smooth and nicely rounded corner on the inside of the joint.
A floating of solder not only prevents liquid foodstuffs from penetrating the joint and becoming rancid, but also provides a radiused corner which can easily be kept clean by simply wiping with a damp cloth.
Source: http://local.ecollege.ie/Content/APPRENTICE/liu/sheetmetal_notes/module3/Soft%20Soldering%20Lap%20Joint_M3_U8.doc
Web site to visit: http://local.ecollege.ie/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes